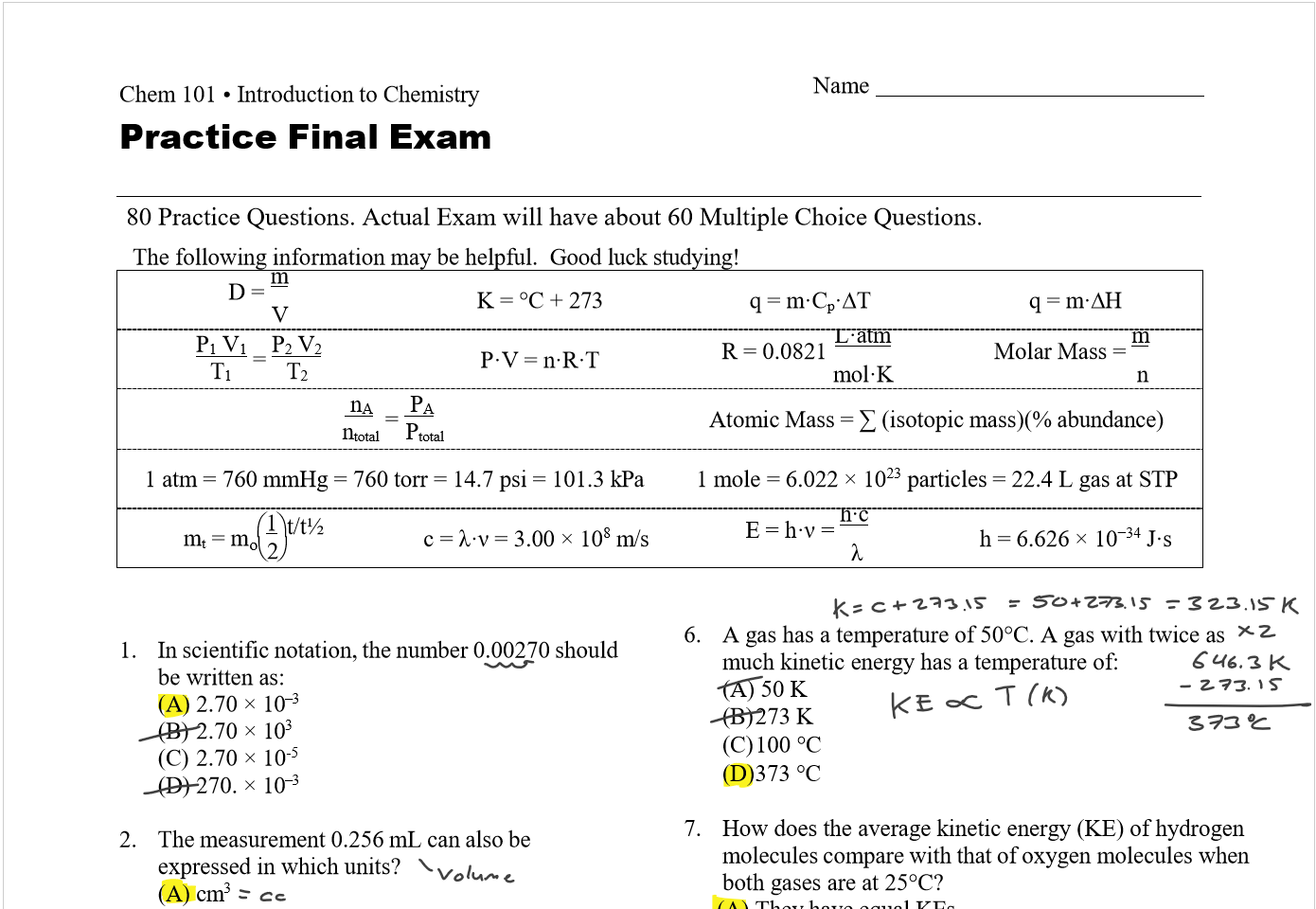
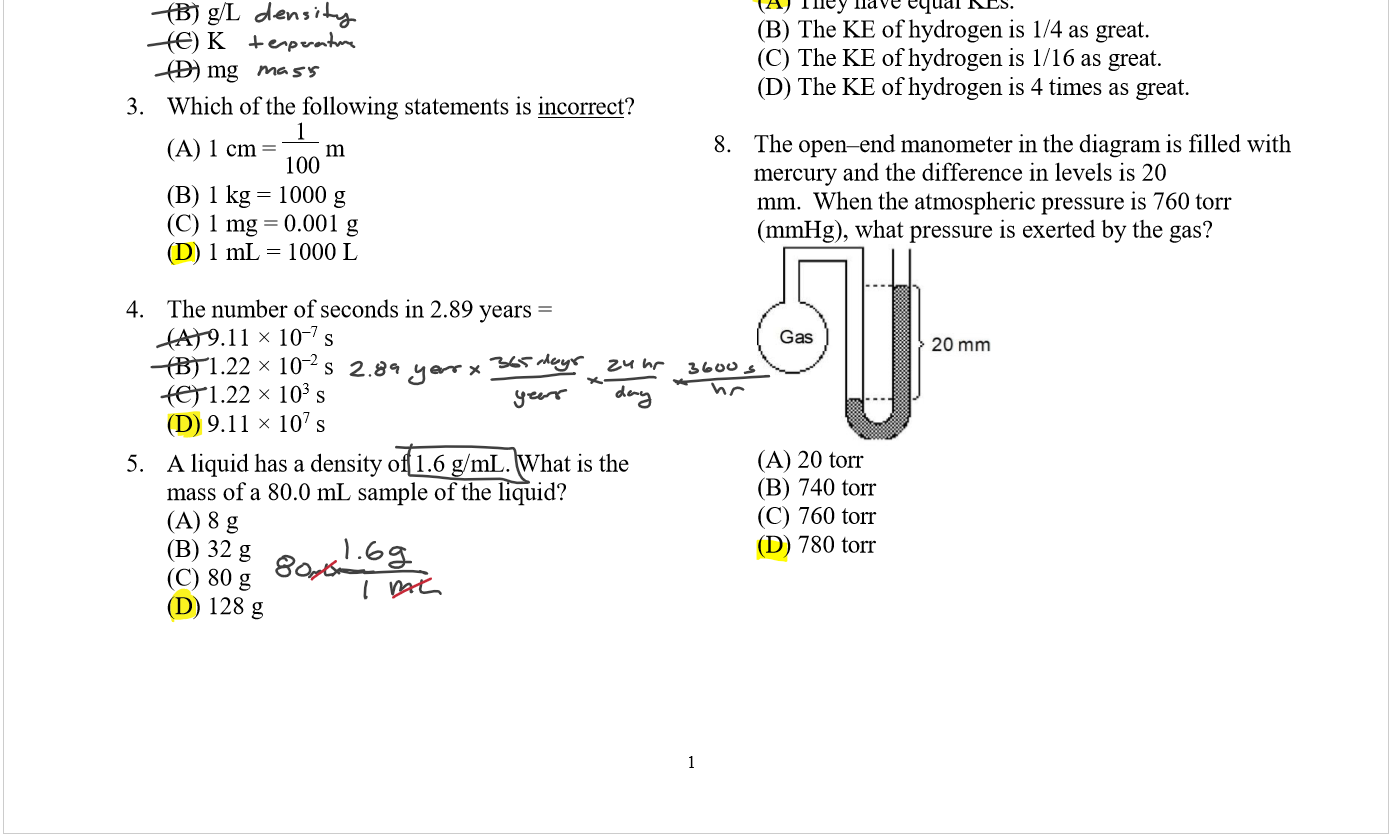
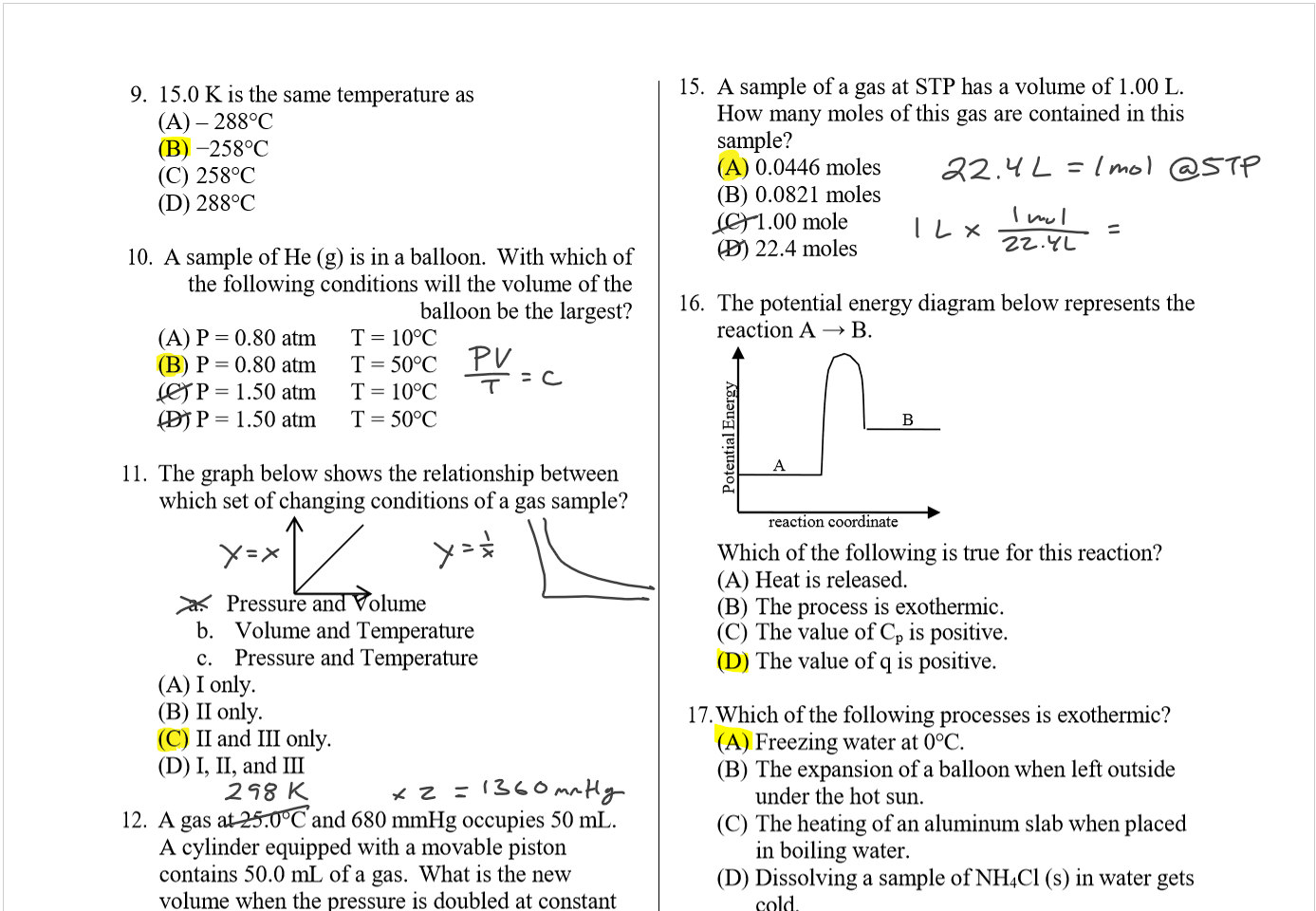
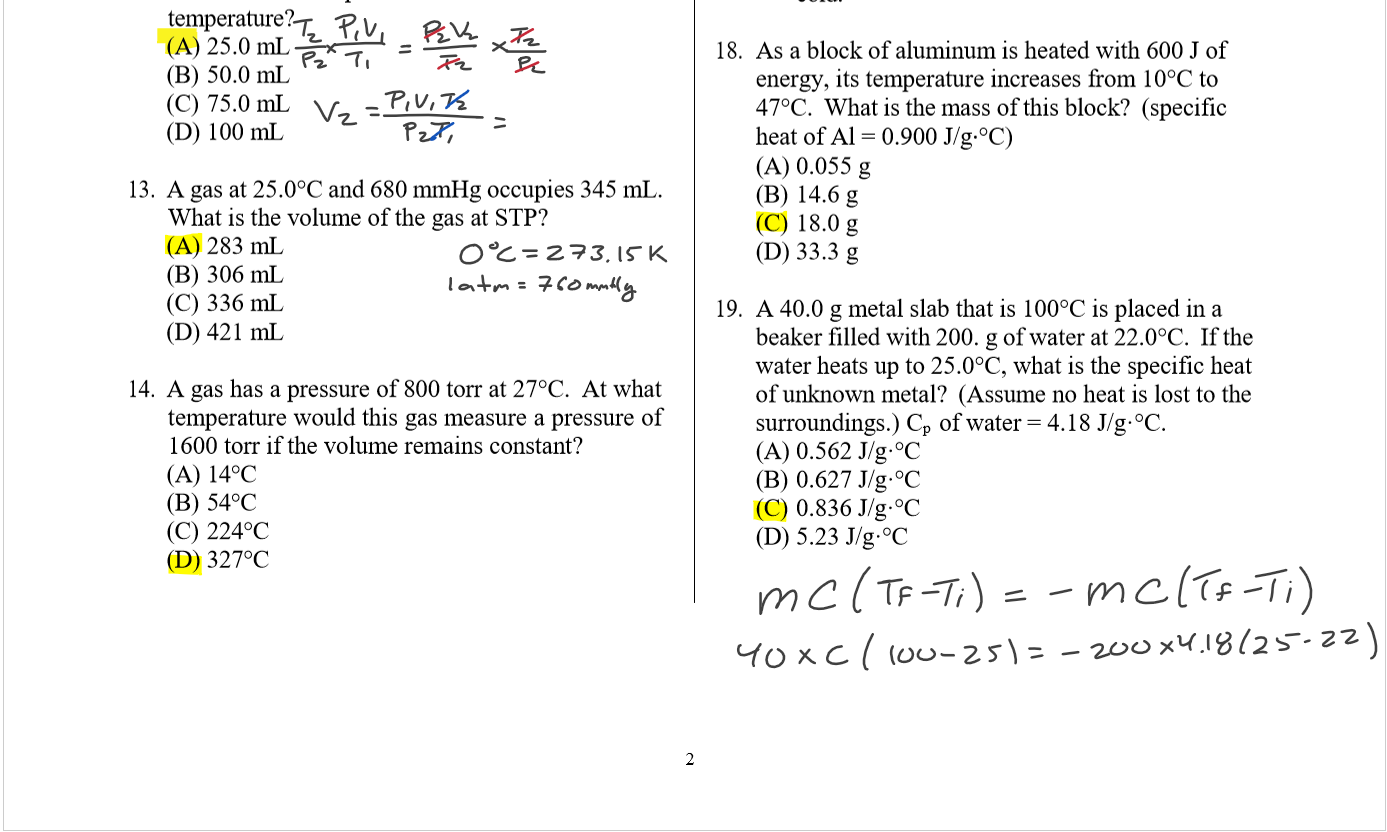
Lecture 26 - Final Exam Review
Thursday, April 25, 2024
9:00 AM
Class notes (1-25): https://bricejurban.github.io/CHEM101/ Assignments this week: Reminders:
My CIC (EDUC 107) Hours: Friday 11AM - 1PM Office Hours (SCNC 314 or Zoom): By appointment |
Today (4/23)
Tuesday (4/30) configuration handout, VSEPR Handout, solubility chart, stoichiometry map, and the midterm 4 equation sheet
|
![Embedded file printout 101 Final Exam Practice Test_4.pdf Machine generated alternative text:
37. A radioactive substance has a half-life of 20
31 _ The atomic mass of arsenic is 74_92_ Which ofthe
following is true?
(A) A mole of arsenic has a mass of 74.92 amu
(B) A mole of arsenic has a mass of 74.92
(C) An atom of arsenic has amass of 74.92 g.
(D) There are 7492 atoms of arsenic in one gram
32 _ The element bromine, Br, forms an ion with a
charge of:
33. Which element is diatomic and forms —1 ions?
(A) calcium
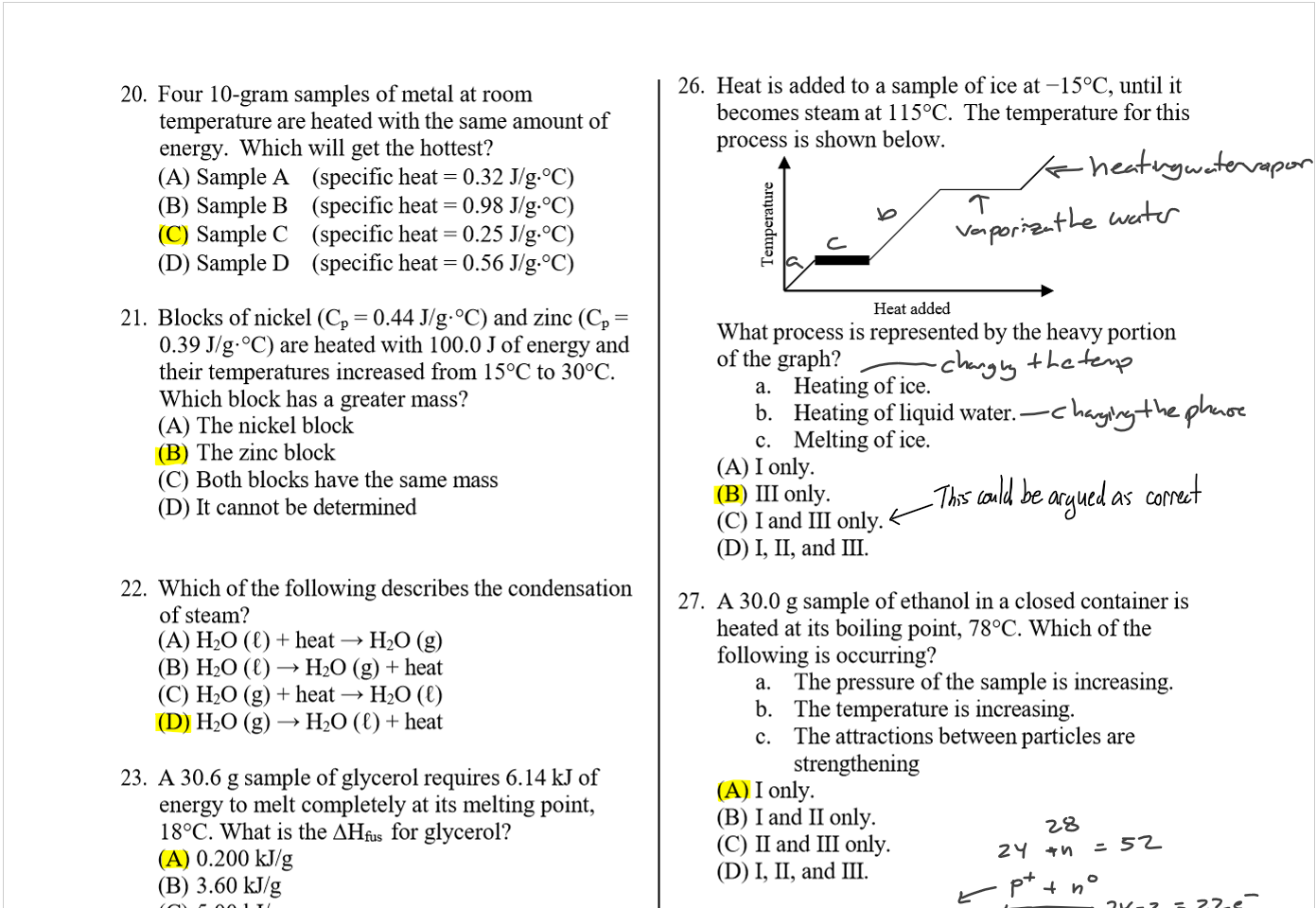
(B) iodine
(C) oxygen
(D) potassium
34 _ Which of the follmving statements refers to an
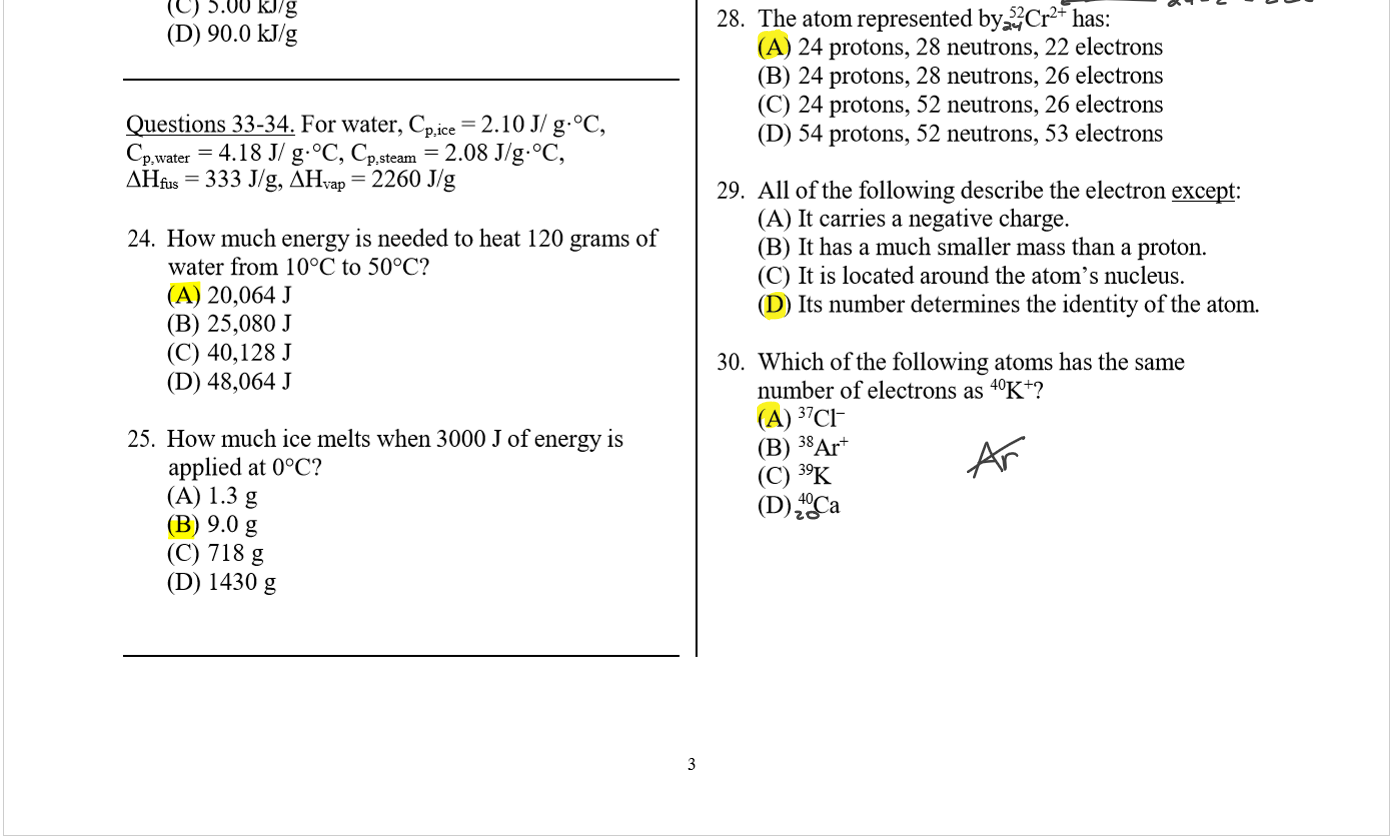
alpha particle ?
(A) a highly energetic form of light.
(B) a high-speed electron
(C) a nucleus of a helium atom_
(D) has the symbol 0 _le-
35 _ When PO undergoes alpha decay, the resulting
isotope is
36. Which symbol completes this nuclear equation?
(A) 0 13
minutes. How long will it take for a 64-gram
sample to decay to 2 grams?
38.
(A) 40 minutes
(B) 60 minutes
(C) 80 minutes
(D) 100 minutes
What is the half-life of this substance?
(A) 4 days
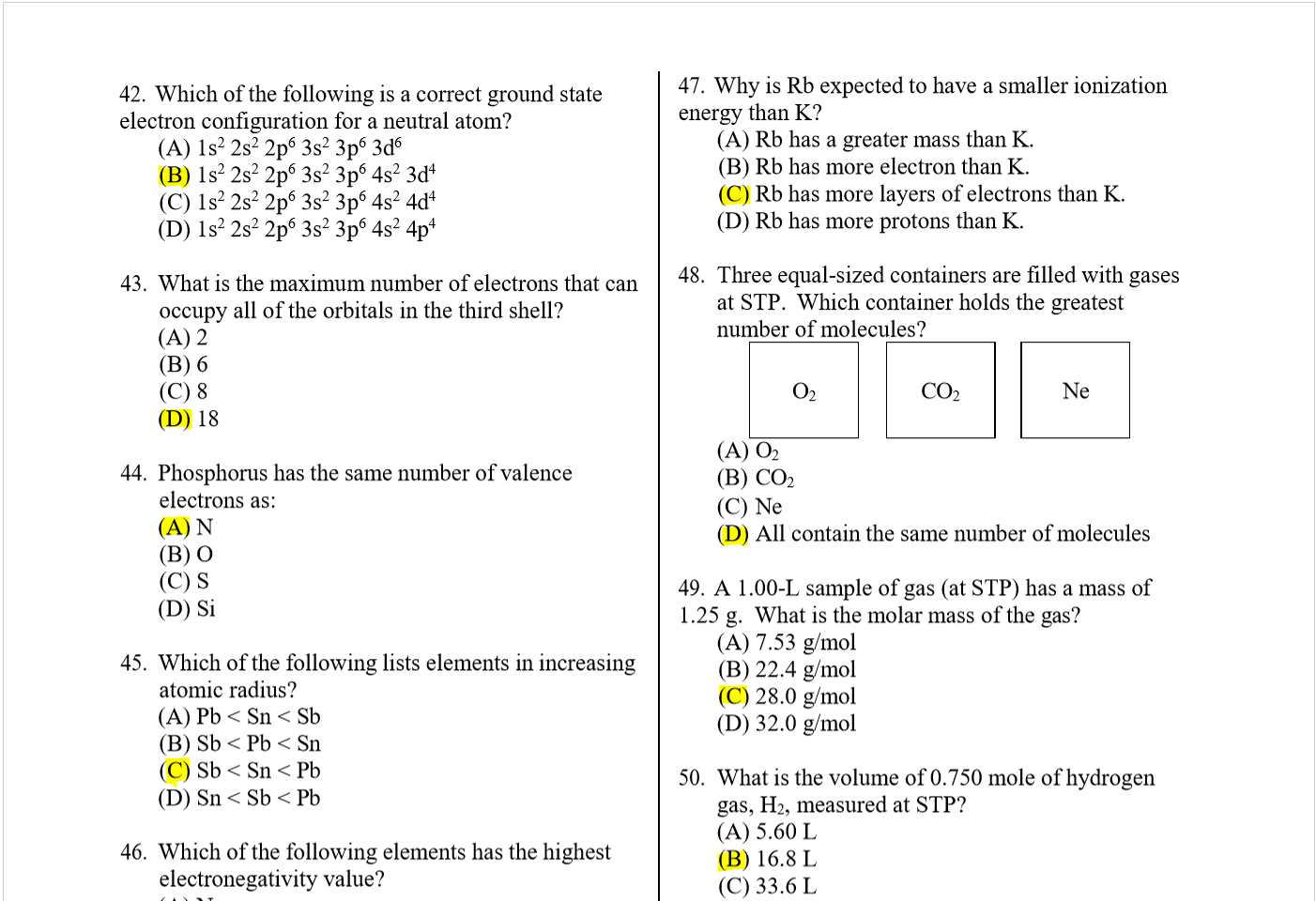
(B) 6 days
(C) 12 days
(D) 24
39. What is the electron configuration for the Te
atom?
40.
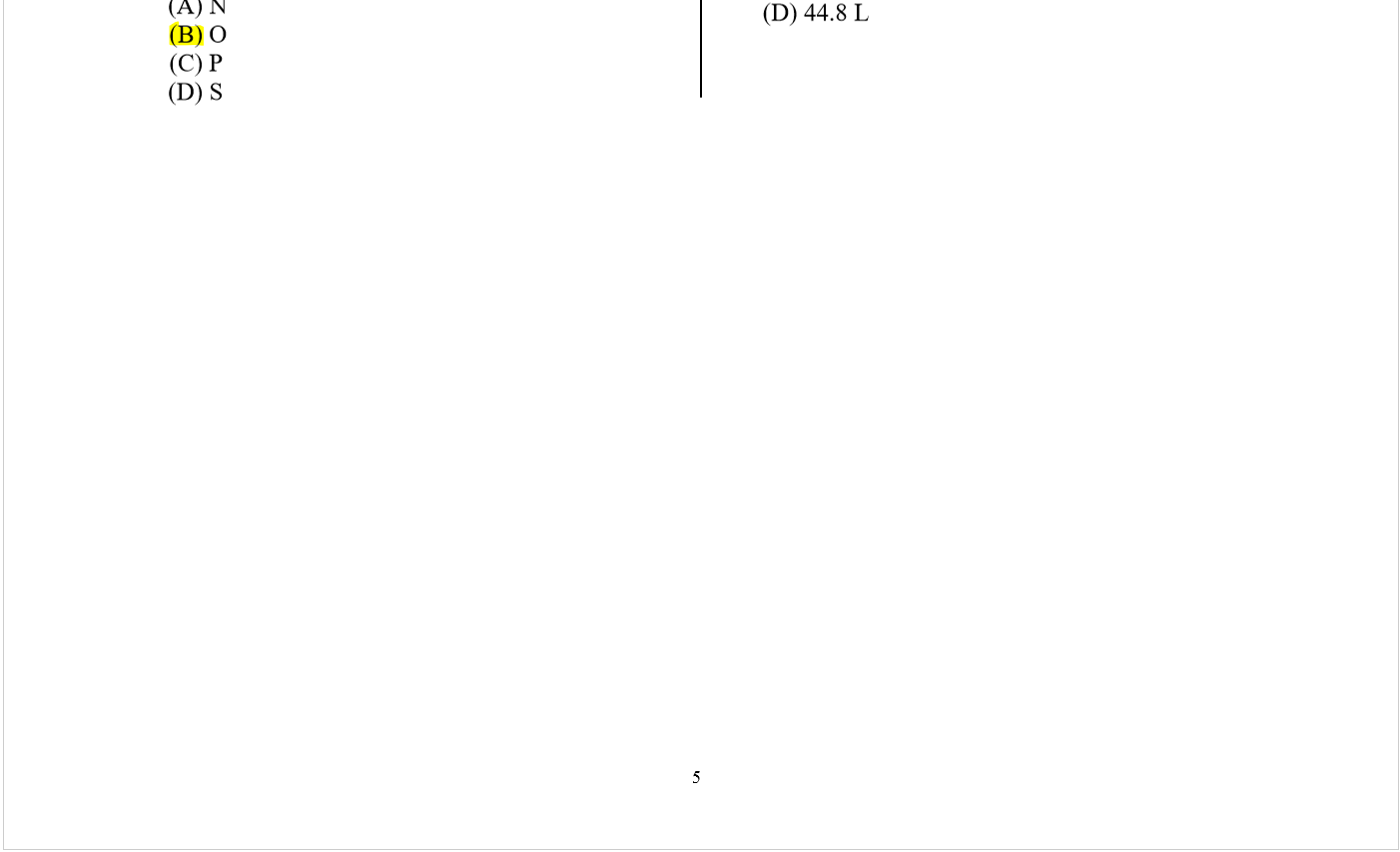
41.
(D) [Kr]5s25pf
The electrons in vanadium (atomic number 23)
that are fanhest from the nucleus occupy the
orbital.
What is the ground state electron configuration of
the As3* ion?
(B) [AT] 4p2
(C) [Ar]4s23d10
(D) [AT] 4s2 3d10 4p3
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Untitled picture
Ink Drawings
Ink Drawings
Ink Drawings](image007.png)
![Embedded file printout 101 Final Exam Practice Test_4.pdf Machine generated alternative text:
37. A radioactive substance has a half-life of 20
31 _ The atomic mass of arsenic is 74_92_ Which ofthe
following is true?
(A) A mole of arsenic has a mass of 74.92 amu
(B) A mole of arsenic has a mass of 74.92
(C) An atom of arsenic has amass of 74.92 g.
(D) There are 7492 atoms of arsenic in one gram
32 _ The element bromine, Br, forms an ion with a
charge of:
33. Which element is diatomic and forms —1 ions?
(A) calcium
(B) iodine
(C) oxygen
(D) potassium
34 _ Which of the follmving statements refers to an
alpha particle ?
(A) a highly energetic form of light.
(B) a high-speed electron
(C) a nucleus of a helium atom_
(D) has the symbol 0 _le-
35 _ When PO undergoes alpha decay, the resulting
isotope is
36. Which symbol completes this nuclear equation?
(A) 0 13
minutes. How long will it take for a 64-gram
sample to decay to 2 grams?
38.
(A) 40 minutes
(B) 60 minutes
(C) 80 minutes
(D) 100 minutes
What is the half-life of this substance?
(A) 4 days
(B) 6 days
(C) 12 days
(D) 24
39. What is the electron configuration for the Te
atom?
40.
41.
(D) [Kr]5s25pf
The electrons in vanadium (atomic number 23)
that are fanhest from the nucleus occupy the
orbital.
What is the ground state electron configuration of
the As3* ion?
(B) [AT] 4p2
(C) [Ar]4s23d10
(D) [AT] 4s2 3d10 4p3
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image008.png)
![Embedded file printout 101 Final Exam Practice Test_6.pdf Machine generated alternative text:
The following information may be helpfiul for the next set of questions.
l_ox 10-14
K=OC+273
P-V=nR-T
14
= 14.7 psi = 101.3 kPa
0.0821 L.atm/
mol-K
E] I O-PH
= V,-Md
Molar Mass =m/n
POH = -logCOff]
[Off] = I O-POH
1 atm = 760 mmHg
760 torr
1 mole = 6.022 x 1023 panicles = 224 L gas at STP
56. mlich one of the following is the correct
formula for calcium phosphate?
51 _ Bonds between which ofthe following elements is
expected to be most polar?
(D) S-Br
52. Which type ofbond is described as "alattice of
positive ions in a sea of electrons"?
(A) co\.alent
(B) hydrogen
(C) ionic
(D) metallic
53. The compound Oü-14)2COy contains how many
atoms of hydrogen?
54. An un_known element X forms a salt with
the formula X02_ Which of the following
could be X?
(B) cal*
(D) sn4*
55. mlich ofthe following name is correctly
paired with its formula?
57 _
58.
(C) CaP04
'What is the name of the compound N203?
(A) dinitride trioxide
(B) dinitrogen trioxide
(C) nitrate
(D) nitrogen oxide
What is the percent of carbon m barium carbonate,
(A) Carbonate
(B) Chloride
(C) F erric
(D) Nitrate
co
CIO -
NO
BaC03 (molar mass = 197.3 g/mol)?
(C) 140%
(D) 20 0%
59 _ How many moles of hydrogen cyanide, HCN, are
contained in 9.00 grams ofHCN? (molar mass = 2703
g/mol)
(A) 0.333
(B) 0 900
60. How many molecules are in 2.00 x I(F2 moles of
carbon tetrachloride, CC14? (molar mass = 154 g/mol)
(A) 1.20 x 1023
(B) 3.01 x
(C) 1023
(D) 1.20 x 1022
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image011.png)
![Embedded file printout 101 Final Exam Practice Test_6.pdf Machine generated alternative text:
The following information may be helpfiul for the next set of questions.
l_ox 10-14
K=OC+273
P-V=nR-T
14
= 14.7 psi = 101.3 kPa
0.0821 L.atm/
mol-K
E] I O-PH
= V,-Md
Molar Mass =m/n
POH = -logCOff]
[Off] = I O-POH
1 atm = 760 mmHg
760 torr
1 mole = 6.022 x 1023 panicles = 224 L gas at STP
56. mlich one of the following is the correct
formula for calcium phosphate?
51 _ Bonds between which ofthe following elements is
expected to be most polar?
(D) S-Br
52. Which type ofbond is described as "alattice of
positive ions in a sea of electrons"?
(A) co\.alent
(B) hydrogen
(C) ionic
(D) metallic
53. The compound Oü-14)2COy contains how many
atoms of hydrogen?
54. An un_known element X forms a salt with
the formula X02_ Which of the following
could be X?
(B) cal*
(D) sn4*
55. mlich ofthe following name is correctly
paired with its formula?
57 _
58.
(C) CaP04
'What is the name of the compound N203?
(A) dinitride trioxide
(B) dinitrogen trioxide
(C) nitrate
(D) nitrogen oxide
What is the percent of carbon m barium carbonate,
(A) Carbonate
(B) Chloride
(C) F erric
(D) Nitrate
co
CIO -
NO
BaC03 (molar mass = 197.3 g/mol)?
(C) 140%
(D) 20 0%
59 _ How many moles of hydrogen cyanide, HCN, are
contained in 9.00 grams ofHCN? (molar mass = 2703
g/mol)
(A) 0.333
(B) 0 900
60. How many molecules are in 2.00 x I(F2 moles of
carbon tetrachloride, CC14? (molar mass = 154 g/mol)
(A) 1.20 x 1023
(B) 3.01 x
(C) 1023
(D) 1.20 x 1022
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image012.png)
![Embedded file printout 101 Final Exam Practice Test_7.pdf Machine generated alternative text:
61.1%ich of the following describes the bonds in a
molecule of ethyne, C2H2?
(A) 1 double bond, 2 single bonds
(B) 1 triple bond, 2 single bonds
(C) 2 double bonds, 1 single bond
(D) 3 single bonds
62. The total number of dots draun in the Lewis
structure of nitrogen, N2, is
(B) 10
(C) 14
(D) 16
63. Which set of coefficients balances the equation
for the complete combustion of ethane, C2Hf?
70. If a solution has [Off] — 1.0 x 10-3 M, what is the
__02(g)
64. When equation for the combustion of
propene, C3Ht, is balanced with the Iou-est
uhole-number coefficients, what is the
coefficient of oxygen, 02?
(C) 12
(D) 18
65 Which of the following represents the
dissoclatlon of Ca12 in solution?
(A) ca12 Ca +12
(B) cab Ca2++21-
(C) Ca12 Ca2+ + 12
(D) Cab is insoluble so it does not
dissociate.
66. How many grams of sodium hydroxide pellets,
NaOH, are required to prepare 50.0 mL of a 0.150
solution? [molar mass NaOH = 40.0 g/mol]
(A) 0.300
(C) 300
(D) 200
67. List the following solutions prepared with the
same solute in order of increasing concentration:
L 30.0 g solute in a 240 mL solution
IL 30.0 g solute in a 120 mL solution
Ill. 60.0 g solute in a 120 mL solution
(B) 11 < 1<111
68. A 100 tnL sample of a solution uith a concentration
of 5.00 M is diluted to a new volume of 400 mL with
distilled water. The new concentration will be
(A) 1.25 M
(D) 20.0 M
69. Which chemical is the conjugate base in the
reaction?
+ Off
(B)
(C) NH
(D) Off
(C) 11
(D) 14
71 _ An acid was neutralized by the
following reaction _
NaOH+HC1 NaC1 + H20
This reaction would be classified as
(A) decomposition
(B) double replacement
(C) single replacement
(D) synthesis
72. HBPOY
H3P04+ _ PHA
When the equation above is balanced, the
coefficient for HYPO* is:
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image013.png)
![Embedded file printout 101 Final Exam Practice Test_7.pdf Machine generated alternative text:
61.1%ich of the following describes the bonds in a
molecule of ethyne, C2H2?
(A) 1 double bond, 2 single bonds
(B) 1 triple bond, 2 single bonds
(C) 2 double bonds, 1 single bond
(D) 3 single bonds
62. The total number of dots draun in the Lewis
structure of nitrogen, N2, is
(B) 10
(C) 14
(D) 16
63. Which set of coefficients balances the equation
for the complete combustion of ethane, C2Hf?
70. If a solution has [Off] — 1.0 x 10-3 M, what is the
__02(g)
64. When equation for the combustion of
propene, C3Ht, is balanced with the Iou-est
uhole-number coefficients, what is the
coefficient of oxygen, 02?
(C) 12
(D) 18
65 Which of the following represents the
dissoclatlon of Ca12 in solution?
(A) ca12 Ca +12
(B) cab Ca2++21-
(C) Ca12 Ca2+ + 12
(D) Cab is insoluble so it does not
dissociate.
66. How many grams of sodium hydroxide pellets,
NaOH, are required to prepare 50.0 mL of a 0.150
solution? [molar mass NaOH = 40.0 g/mol]
(A) 0.300
(C) 300
(D) 200
67. List the following solutions prepared with the
same solute in order of increasing concentration:
L 30.0 g solute in a 240 mL solution
IL 30.0 g solute in a 120 mL solution
Ill. 60.0 g solute in a 120 mL solution
(B) 11 < 1<111
68. A 100 tnL sample of a solution uith a concentration
of 5.00 M is diluted to a new volume of 400 mL with
distilled water. The new concentration will be
(A) 1.25 M
(D) 20.0 M
69. Which chemical is the conjugate base in the
reaction?
+ Off
(B)
(C) NH
(D) Off
(C) 11
(D) 14
71 _ An acid was neutralized by the
following reaction _
NaOH+HC1 NaC1 + H20
This reaction would be classified as
(A) decomposition
(B) double replacement
(C) single replacement
(D) synthesis
72. HBPOY
H3P04+ _ PHA
When the equation above is balanced, the
coefficient for HYPO* is:
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image014.png)
![Embedded file printout 101 Final Exam Practice Test_8.pdf Machine generated alternative text:
73.1%en solutions of potassium sulfate and
calcium bromide are combined, which of the
following precipitates?
(A) KBr
(B) Cas
(C) CaS04
(D) There is no precipitate.
74. Which of the following are products
uhen magnesium metal is placed in
hydrochloric acid?
(D) MgC12
75 _ 1%at mass of sulfiLr dioxide, SO: (64.0 g/mol), is
produced when 245 g of sulfuric acid, H2S04 (98.0 V"
mol) reacts completely Mith zinc metal according to the
balanced equation below?
ztl+ 2 H2S04 -i ZnS04 + SO +2 HIC)
(A) 64.0 g
(B) 80.0 g
(C) 128 g
(D) 160 g
76. At STP, how many liters of oxygen gas react with
4.00 moles of PHS according to this equation?
4PH3 (g) +602 (g) 6H20 (0 +P40, (s)
(A) 32.0
(B) 896
(C) 134
(D) 146
77 _ How many moles ofFeS2 are required to produce 64
grams of SOI according to the balanced equation IRIow?
4FeS2 (s)+ 11 02 (s) (g)
(A) 0.40
(B) 0.50
(C) 3.2
(D) 4.5
78 _ Consider the reaction: 2 NO(g) + 02(g) 2 NOZ (g)
At equilibrium, [NO] = 0.10 NL [02] = 0.10 M, and [NO] —
0.010 NL This reaction is considered:
(A) Reactant-favored
(B) Product-favored
(C) Neither reactant nor product-favored
(D) It cannot be determined
79. Consider the reaction:
2 soz(g) + 02(g) t; 2 soy(g) + heat
which of the following umlld shift the reaction to produce
more S02(g)?
(A) Add 02 to the reaction mixture
(B) Increase the volume of the container.
(C) Lowering the temperature.
(D) Remove S03(g) to the reaction mixture.
80. In the reaction
heat + 2 HBr(g) H2(g) +
Under which conditions would produce the most Br2(t)?
(A) High temperature, low pressure
(B) High temperature, high pressure
(C) Low temperature, low pressure
(D) Low temperature, high pressure
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image015.png)
![Embedded file printout 101 Final Exam Practice Test_8.pdf Machine generated alternative text:
73.1%en solutions of potassium sulfate and
calcium bromide are combined, which of the
following precipitates?
(A) KBr
(B) Cas
(C) CaS04
(D) There is no precipitate.
74. Which of the following are products
uhen magnesium metal is placed in
hydrochloric acid?
(D) MgC12
75 _ 1%at mass of sulfiLr dioxide, SO: (64.0 g/mol), is
produced when 245 g of sulfuric acid, H2S04 (98.0 V"
mol) reacts completely Mith zinc metal according to the
balanced equation below?
ztl+ 2 H2S04 -i ZnS04 + SO +2 HIC)
(A) 64.0 g
(B) 80.0 g
(C) 128 g
(D) 160 g
76. At STP, how many liters of oxygen gas react with
4.00 moles of PHS according to this equation?
4PH3 (g) +602 (g) 6H20 (0 +P40, (s)
(A) 32.0
(B) 896
(C) 134
(D) 146
77 _ How many moles ofFeS2 are required to produce 64
grams of SOI according to the balanced equation IRIow?
4FeS2 (s)+ 11 02 (s) (g)
(A) 0.40
(B) 0.50
(C) 3.2
(D) 4.5
78 _ Consider the reaction: 2 NO(g) + 02(g) 2 NOZ (g)
At equilibrium, [NO] = 0.10 NL [02] = 0.10 M, and [NO] —
0.010 NL This reaction is considered:
(A) Reactant-favored
(B) Product-favored
(C) Neither reactant nor product-favored
(D) It cannot be determined
79. Consider the reaction:
2 soz(g) + 02(g) t; 2 soy(g) + heat
which of the following umlld shift the reaction to produce
more S02(g)?
(A) Add 02 to the reaction mixture
(B) Increase the volume of the container.
(C) Lowering the temperature.
(D) Remove S03(g) to the reaction mixture.
80. In the reaction
heat + 2 HBr(g) H2(g) +
Under which conditions would produce the most Br2(t)?
(A) High temperature, low pressure
(B) High temperature, high pressure
(C) Low temperature, low pressure
(D) Low temperature, high pressure
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings
Ink Drawings](image016.png)