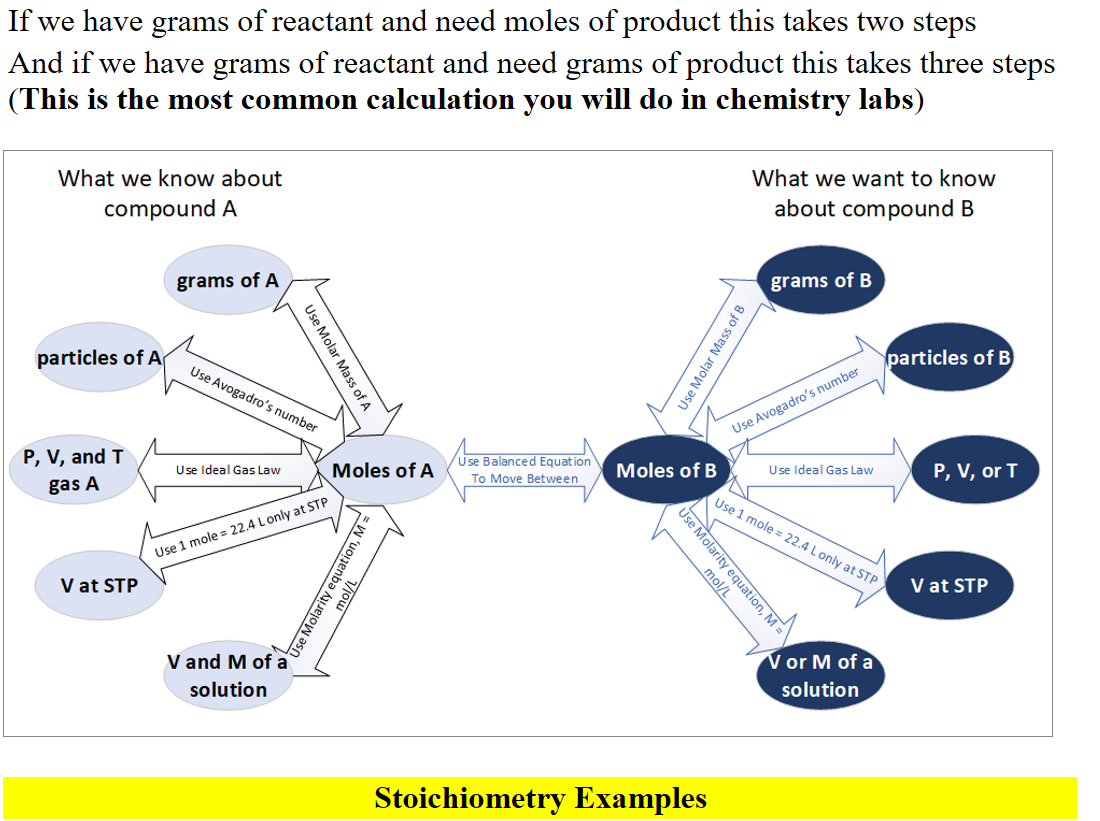
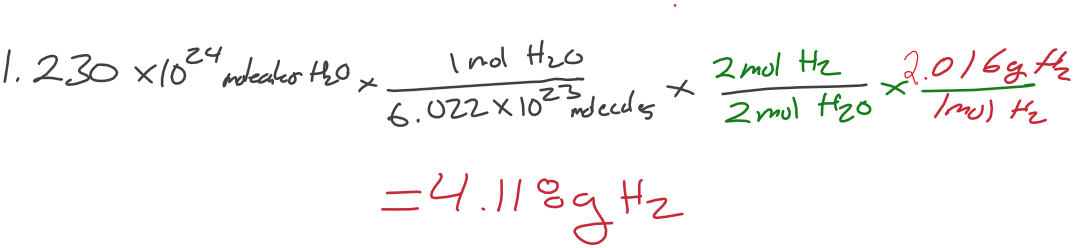Lecture 6 - Moles, Moles, Moles
Thursday, January 25, 2024
11:50 AM




|
|
|||||
|
|
|||||
|
|
|||||
|
|
|||||
|
|
|||||
|
|
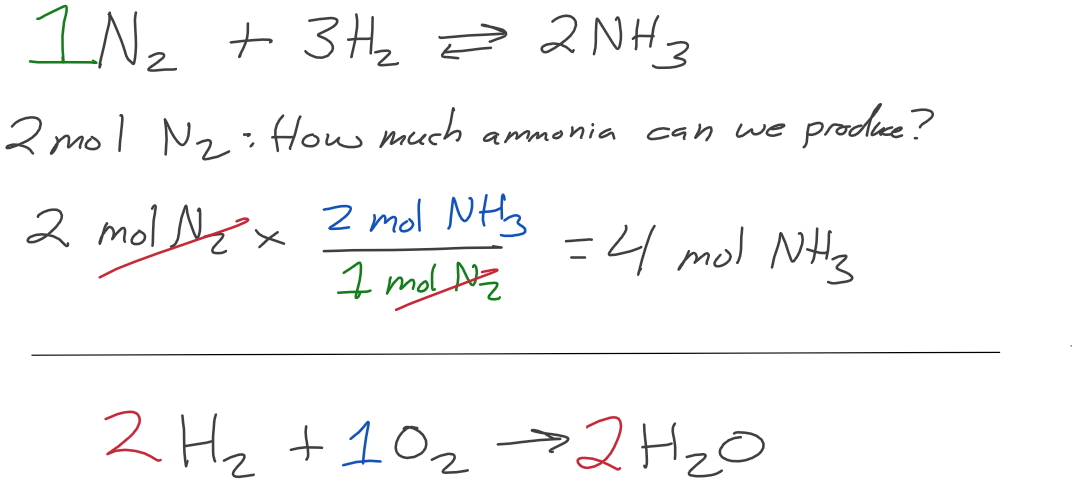
How many moles of water are produced when 5.00 moles of oxygen are used?
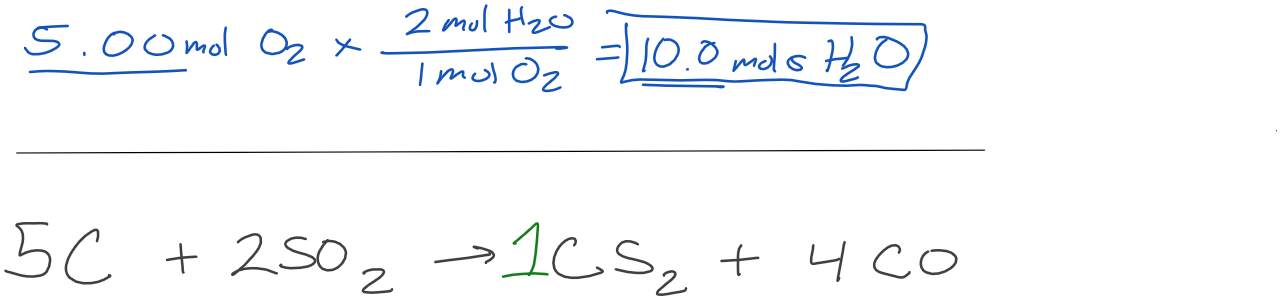
How many moles of SO2 are required to make 125 mols of CS2?
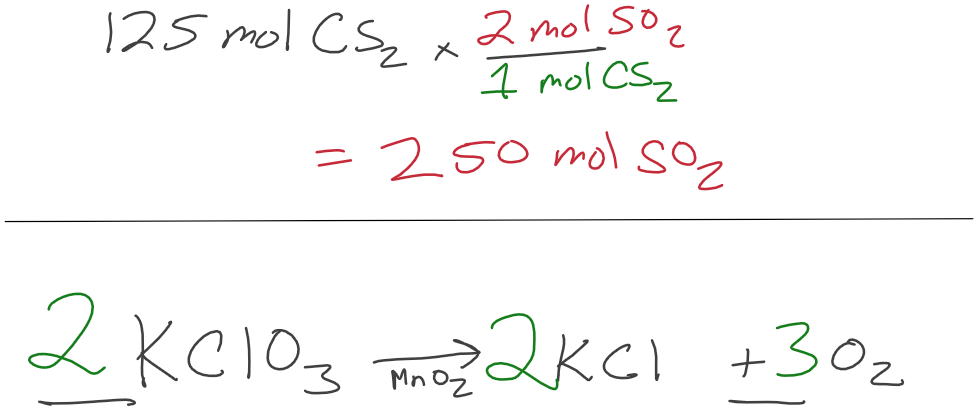
1.50 mol of KClO3 decomposes. How many grams of O2 will be produced?
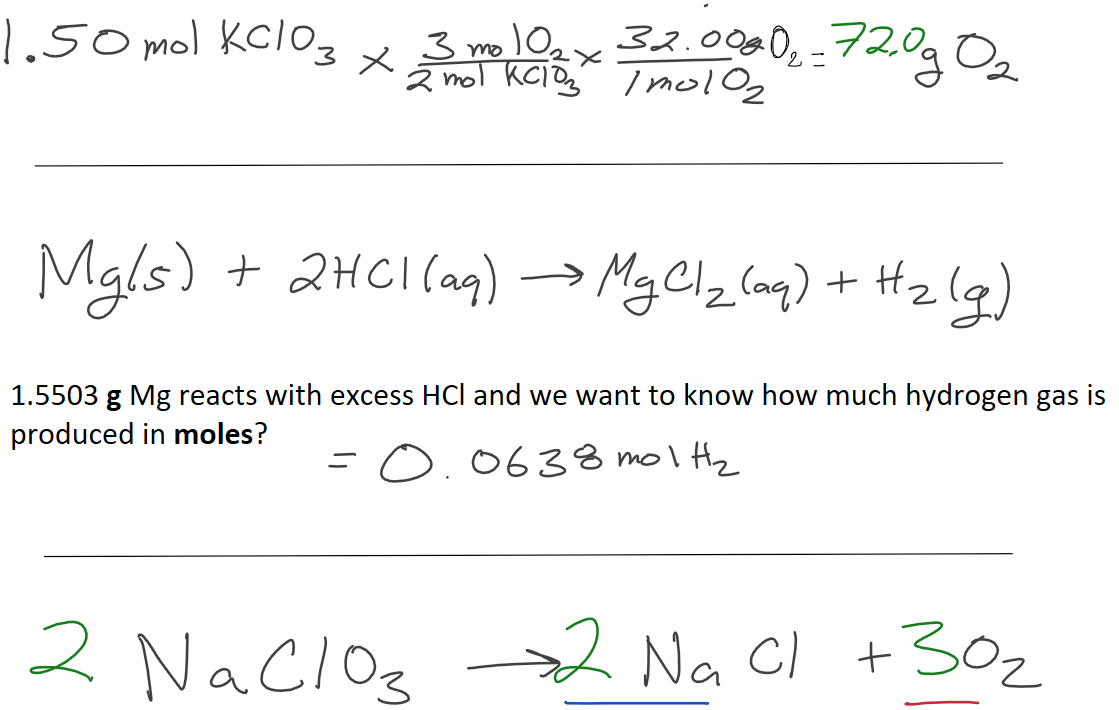
How many grams of NaCl are produced when 80.0 grams of O2 are produced?
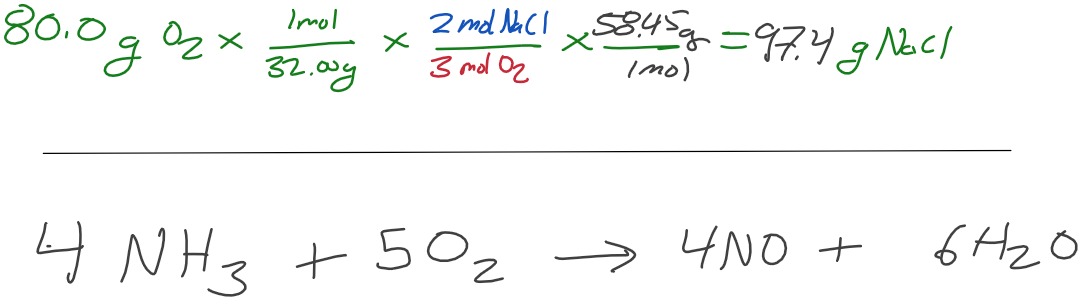
What mass of NO is produced by the reaction of 6.40 grams of oxygen gas?
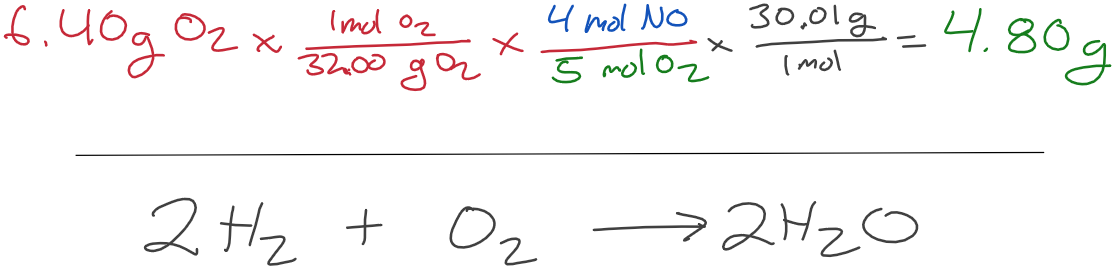
How many grams of H2 are required to produce 1.230 x 1024 molecules of water?